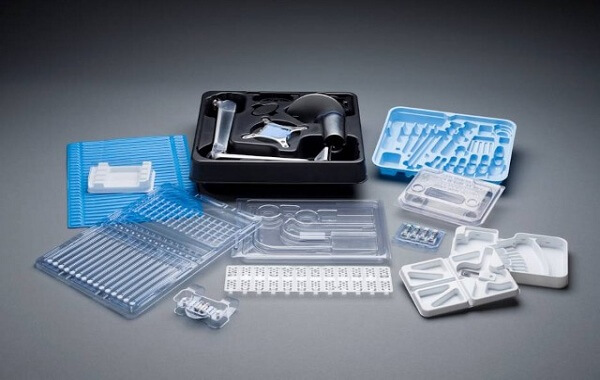
This comprehensive guide delves into the specialized world of cleanroom injection molding, a critical process for industries where microscopic contamination is not an option. We'll define what it is, break down the stringent step-by-step manufacturing process, and explore real-world applications through detailed case studies. For manufacturers considering or optimizing this process, this article provides the essential knowledge on contamination control, material selection, and operational protocols to ensure product integrity and compliance in sensitive sectors like medical and electronics.
Introduction
Why is a standard injection molding machine insufficient for producing a pacemaker casing or a microchip component? The answer lies in invisible threats: particulate, microbial, and molecular contamination. Injection molding in a clean room is not merely molding in a cleaner environment; it is a fully integrated manufacturing philosophy designed to meet the exacting standards of ISO Class 5 to 8 environments. This process safeguards products where even a single speck of dust can lead to device failure, patient harm, or significant financial loss. For manufacturers, mastering this discipline is a gateway to high-value, high-reliability markets.
What is Injection Molding in a Clean Room?
Injection molding in a clean room refers to the process of manufacturing plastic parts within a strictly controlled environment where airborne particulates, temperature, humidity, and pressure are meticulously regulated. The goal is to prevent contamination of the parts during production. It goes far beyond basic cleanliness, involving specialized equipment, stringent operational protocols, and rigorous personnel training.
The core distinction from conventional molding lies in the integration of the molding machine itself into the contamination control strategy. It's not just the room, but the entire process flow—from material handling and drying to part removal and packaging—that is designed for minimal particle generation and maximum control.
- Key Standards: Operations are classified under ISO 14644-1 standards (e.g., ISO Class 7) or the older US FS 209E classes. The required class depends on the application's sensitivity.
- Contaminants Controlled: The process targets particulate contamination (dust, fibers), biological contamination (microbes), and non-volatile residue (NVR) from oils or lubricants.
The Process of Injection Molding in a Clean Room
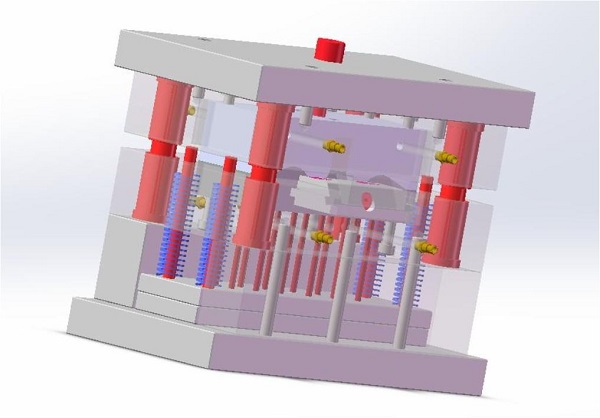
The process is a symphony of controlled variables. It starts with a purpose-built or modified injection molding machine, often with stainless steel surfaces, sealed panels, and low-emission hydraulic systems or all-electric drives to eliminate oil mist. Key components include:
- Cleanroom-Grade Machinery: Machines with smooth, sealed exteriors to prevent particle traps and facilitate cleaning.
- Material Handling Systems: Closed-loop loading and drying systems that prevent material exposure to ambient air.
- Negative Pressure Zones: Creating air pressure differentials around the machine's mold area to contain particles.
- Automated Part Handling: Robots or automated systems for part removal to minimize human intervention and associated contamination.
Step-by-Step Guide
Here is a breakdown of the critical phases in a cleanroom molding cycle:
- Material Preparation & Introduction: Resins are stored and dried in dedicated, controlled anterooms. They are transferred via closed conveying systems directly into the machine hopper, avoiding any manual pouring which can introduce particles and moisture.
- Machine Purge & Setup: Before production, machines undergo a thorough purge and cleaning. Molds are cleaned and installed using cleanroom-compatible protocols. All setup tools are also of cleanroom grade.
- The Molding Cycle in Controlled Atmosphere: The cycle runs within the clean room's laminar airflow. Contamination control is continuous, with air showers and HEPA/ULPA filters constantly scrubbing the air. Process parameters are tightly monitored for consistency.
- Automated Ejection & Handling: Upon ejection, parts are immediately handled by a cleanroom-rated robot. Direct human contact is avoided. The robot typically places parts into clean, sealed containers or onto a conveyor leading to a sealing/packaging station.
- In-Line Inspection & Packaging: Parts may undergo in-line visual or automated optical inspection. Packaging occurs immediately, often within an adjacent cleanroom zone, using static-dissipative or sterile packaging materials.
| Process Stage | Conventional Molding | Cleanroom Molding | Key Contamination Risk Mitigated |
|---|---|---|---|
| Material Loading | Manual pouring from bags | Closed-loop, automated conveying | Particulate introduction, moisture absorption |
| Machine Environment | Open factory floor | ISO-classified room with HEPA filtration | Airborne particles, microbes |
| Part Removal | Manual or robot | Cleanroom-rated robot with smooth surfaces | Human-borne contaminants (skin cells, fibers) |
| Post-Processing | Bench trimming, assembly | In-line, automated or in separate controlled zone | Particulate generation from secondary operations |
Case Studies
Medical Device Manufacturing
A leading contract manufacturer needed to produce a complex fluidic connector for a diagnostic device. The part required ISO Class 7 (Class 10,000) conditions and biocompatibility (ISO 10993).
The Challenge: Any particulate contamination inside the fluidic channels could clog the device and cause diagnostic errors. Microbial contamination was unacceptable.
The Cleanroom Molding Solution:
- An all-electric injection press was installed in an ISO Class 7 suite to eliminate hydraulic oil risk.
- A dedicated, validated resin drying system was used, with dew point monitoring.
- A vision-guided robot removed parts and placed them directly into clean, labeled trays within a laminar flow hood.
- 100% automated leak and flow testing was integrated post-mold, inside the cleanroom.
The Outcome: The manufacturer achieved a zero-defect rate for contamination-related failures over 5 million parts, passing all customer audits and FDA regulatory scrutiny.
Electronic Component Production
A semiconductor company required precise overmolded connectors for sensor modules. The parts demanded ESD safety and ultra-low outgassing to prevent corrosion on nearby circuits.
The Challenge: Static discharge could fry sensitive chips. Silicone or oil residues (non-volatile residue) outgassing from the plastic could condense on optical sensors.
The Cleanroom Molding Solution:
- The molding cell was established in an ISO Class 8 room with controlled humidity to manage static.
- Machines and tools were equipped with full ESD protection.
- A specialty, low-outgassing LCP (Liquid Crystal Polymer) resin was selected and handled in a nitrogen-purged system.
- Parts were packaged in static-shielding bags within seconds of ejection.
The Outcome: The overmolded connectors exhibited no measurable outgassing in thermal vacuum tests, and the yield for the final sensor assembly increased by 15% due to reduced electrostatic damage.
Yigu Technology's View
At Yigu Technology, we view cleanroom injection molding as a total system, not just a location. Our experience shows that success hinges on three pillars:
- Prevention Over Remediation: The cost of preventing contamination is always lower than the cost of sorting, scrapping, or facing field failures. This philosophy drives investment in automated part handling and sealed machinery from the outset.
- Material Mastery is Critical: The cleanroom process starts with the resin pellet. Material selection for biocompatibility and low volatility, coupled with supremely controlled drying and handling, is non-negotiable. You cannot mold a clean part from a contaminated material.
- Data is Your Compliance Shield: Comprehensive monitoring and data logging—of room particulates, machine parameters, and operator access—provide the objective evidence required for medical device or aerospace validation (e.g., FDA 21 CFR Part 820, ISO 13485). This documented control is as valuable as the physical parts produced.
The trend is moving towards "lights-out" automation in cleanrooms, further removing the human variable. For manufacturers, the decision to implement cleanroom molding should be a strategic one, opening doors to markets where quality, reliability, and traceability command a premium.
FAQ
What is the difference between a clean room and a white room for injection molding?
A white room is a controlled space with basic cleanliness, often used for assembly. A clean room has a legally defined, monitored, and certified level of airborne particulate control (per ISO standards), with strict protocols for gowning, material flow, and equipment. All cleanrooms for molding are controlled environments, but not all controlled environments are certified cleanrooms.
How much more expensive is cleanroom injection molding compared to standard molding?
Costs can be 20% to 100%+ higher. This includes capital expenditure for certified rooms and compliant machinery, ongoing operational costs for environmental monitoring and maintenance, higher-grade materials, and extensive documentation for validation and quality control. However, this cost is essential for regulatory compliance and risk mitigation in critical industries.
Can any plastic resin be used in cleanroom molding?
Technically yes, but not all should be. Material selection is vital. Resins must be chosen for low particle generation, low outgassing, and often for specific properties like biocompatibility or ESD safety. Hygroscopic materials (like Nylon, PC) require exceptionally controlled drying to prevent moisture-related defects and microbial growth.
What ISO class is typically required for medical device molding?
Most medical device manufacturing for implants or critical components requires ISO Class 7 (Class 10,000) or cleaner for the molding operation. Secondary operations like assembly and packaging may occur in ISO Class 8 or 7. The final requirement is driven by the device's risk classification and the manufacturer's Quality Management System (QMS).
Contact Yigu Technology for Custom Manufacturing.
Navigating the complexities of injection molding in a clean room requires a partner with proven expertise and a systematic approach. Yigu Technology specializes in providing turnkey, validated cleanroom molding solutions for the most demanding applications in the medical, electronics, and semiconductor industries.
From initial design for manufacturability (DFM) advice to full-scale production in our certified facilities, we ensure your project meets the highest standards of contamination control, precision, and regulatory compliance. Let's discuss how to bring your sensitive component to market with unwavering reliability.
Contact us today for a confidential consultation on your custom manufacturing needs.